

In this experiment, you will separate the components of a commercial headache powder via an extractive process. This separation will be accomplished by taking advantage of the fact that each component contains different functional groups which will react differently when treated with a specific reagent.
| concentration of A in S (g/mL) |
| concentration of A in S' (g/mL) |
The KD value is generally > 1; therefore, S is the solvent in which A has the greatest solubility. The KD may be used to evaluate the effectiveness of an extracting solvent and to plan an extraction. An extracting solvent should be immiscible, have a favorable KD, be nonreactive (with the exception of aqueous solutions of acids and bases) and be easily separated from solute. Some commercial headache remedies contain aspirin as well as caffeine, salicylamide and/or acetaminophen.

Figure 1
Acetylsalicylic acid, Aspirin, is an organic acid; therefore, it is soluble in an organic solvent (diethyl ether), but will react with a basic reagent (:B) such as sodium hydroxide or sodium bicarbonate to produce the conjugate base of the acid. The conjugate base is a salt and is water soluble; therefore, it is removed from the organic solvent layer. Reacidification of this basic aqueous layer will regenerate the organic acid, which will precipitate from the aqueous solution due to the acid's limited solubility in water.
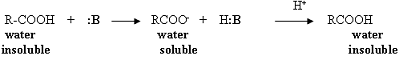
Figure 2
Caffeine is an amine; therefore, it has a basic nitrogen that will react with a proton source such as hydrochloric acid. Reaction with the acid produces the conjugate acid of the amine (an ammonium ion) which is a salt and is water soluble (recall the ammonium ion from General Chemistry). Adding base to the acidic aqueous layer will regenerate the water insoluble amine.
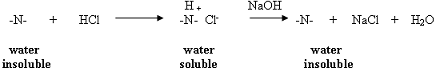
Figure 3
The amide, salicylamide, is neither acidic nor basic enough to react; therefore, it will remain soluble in the organic solvent throughout the extraction process. One of the most commonly used pieces of equipment in the Organic lab is the Separatory Funnel, or "Sep Funnel." Many reactions are completed by separating an aqueous layer from an organic layer. Many reactions require that a component be removed by acid-base extraction. The isolation of useful compounds from naturally occurring materials is a common organic process. All of these can be accomplished by means of a 'Sep Funnel.' As shown below, the Sep Funnel is a pear shaped glass device equipped with a stopcock at the bottom and a stopper to close the top opening. Sep Funnels come in all sizes from about 50 mL to 5 L or larger. They are all very FRAGILE. The Sep Funnel acts like a cocktail shaker. With the stopcock closed, ingredients are added through the top. The stopper is securely placed, and the contents are shaken. While the Sep Funnel is being shaken, the stopper must be held securely in place and the stopcock must be tightly shut. (Your TA will demonstrate the 'art' of securing the stopper in the palm of your hand while shaking the Sep Funnel.) Quite often, the components develop some pressure on being shaken. This pressure must be carefully relieved by slowly opening the stopcock while the Sep Funnel is inverted. Of course, the Sep Funnel must be 'aimed' away from any nearby person in case the pressure is larger than expected.
Figure 4
After the Sep Funnel has been shaken a few times and the pressure relieved once or twice, the Sep Funnel is placed upright in an iron ring, and the stopper is removed. When the two layers are clearly separated, the lower layer may be carefully drained into an Erlenmeyer flask by slowly opening the stopcock.
Caution:
Allow the liquid to drain slowly so that the layers remain clearly separated and a vortex does not develop.
If a second extraction is needed, the layer to be extracted is placed in the Sep Funnel and a new portion of the extracting liquid is added, and the shaking, venting, process is repeated. This is commonly encountered during the "work up" of a chemical reaction, when the organic layer is washed with several different aqueous solutions. Reminder — the stopcock must be cleaned before and after each use. When not in use the stopcock is left clean and assembled, but very loose fitting. The plastic will distort if left tightly held in the glass part. When in use, the stopcock nut must be tightened to keep the stopcock securely held in the glass.

Figure 5
In a 50 mL Erlenmeyer flask, dissolve 1 g of headache powder (the contents of one packet) in 20 mL of diethyl ether. The headache powder contains some binders which are insoluble in ether. All of the powder may not disolve, but this is not a problem.
Make sure the stopcock on the separatory funnel is closed. Pour the solution into the separatory funnel.Use a fresh 5 mL of diethyl ether to transfer the remaining contents of the Erlenmeyer flask to the separatory funnel.
Measure approximately 20 mL of 3 M HCl. Transfer to your Erlenmeyer flask to dissolve the remaining solid. Transfer the 3 M HCl to the separatory funnel. Stopper the funnel, invert the funnel, and release the pressure by opening the stopcock.Continue extraction with ether by shaking, inverting and venting until no audible or visible gas emerges (i.e. nothing comes out of the stopcock when releasing pressure).
Place the separatory funnel in an iron ring on a ring stand and remove the stopper immediately.NOTE: The determination of which layer is organic and which is aqueous is easily accomplished by knowing the densities of the solvents used. Also, one can add a drop of water to the separatory funnel and observe whether the droplets dissolve in the upper layer, or pass through the lower.
Remove the aqueous layer (which is it, top or bottom?) into a labeled beaker. Be careful and slow to dropwise flow as the level of the layers lowers in the funnel. Be sure to leave a drop of the bottom layer in the seperatory funnel.
For treatment of this layer, refer to the section below labeled HCl Layer.Rinse your beaker with 1-2 mL of 5% Sodium Bicarbonate and discard the solution. This is to prevent neutralization of the Sodium Bicarbonate. This is called conditioning.
Next, extract the ether layer with 20 mL of a 5% sodium bicarbonate (NaHCO3) solution. By repeating steps 7-10. (Care must be taken in this step, since pressure buildup is possible from Sodium Bicarbonate.)
One member of this group can work with this solution starting with instructions below for Bicarbonate Extract.
The ether solution is finally washed with 20 mL of a saturated sodium chloride solution in the seperatory funnel. Repeat steps 7-9.
Allow the layers to separate, discard the aqueous layer (make sure which is which). Allow a drop of the top layer to transfer to your beaker.
Transfer the ether solution to a clean, dry, labeled 50 mL Erlenmeyer flask.Add a small amount (enough to cover the bottom of the flask) of anhydrous sodium sulfate (Na2SO4) to the ether solution to absorb any residual water. You can watch a short video that describes this drying process.
Allow the drying process to occur for about 5 minutes.Pour (or filter through a funnel with a small cotton plug) the ether solution into a clean 50 mL Erlenmeyer Flask.
In the bench hood, place the Erlenmeyer flask with the ether layer on a hotplate on a setting that will begin to boil the ether.
Boil off the liquid until there is 1-2 mm of liquid left and remove the flask from the hotplate. The solid that remains is crude salicylamide.
Weigh the salicylamide and determine the sample's melting point.Note: This solution contains the conjugate acid of caffeine. Since the majority of powders contain a small portion of caffeine, isolation will not be performed.
The solution should be neutralized by the gradual addition of 6 M NaOH and discarded in the appropriate waste container. (Recall: How much 6M NaOH will you need to neutralize 20 mL of 3 M HCl?)
While still in an ice bath, carefully acidify this solution by slowly adding the 6 M HCl dropwise until no additional acetylsaliylic acid solid is produced.